Hidrawear
Adhesive-free dressing retention system for wounds in hard-to-dress anatomical areas
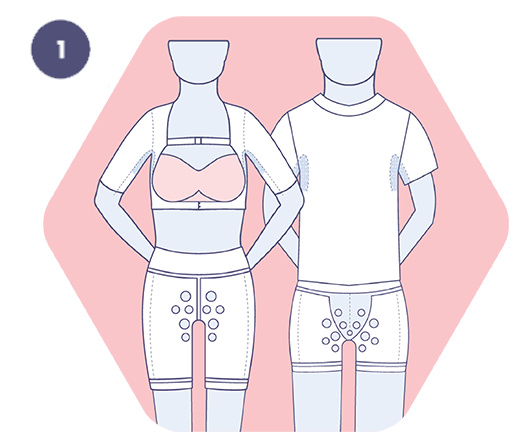
HidraWear is engineered for use in patients with Hidradenitis Suppurativa (HS) and other wounds located in regions difficult to dress (e.g. axilla, groin, buttocks). It utilises a non-adhesive base layer with an external fastening mechanism to hold dressings securely, eliminating the need for skin adhesives and reducing trauma and discomfort during dressing changes.¹
Product Benefits
Made specifically for managing Hidradenitis Suppurativa flares and difficult-to-dress wounds, HidraWear removes the need for the dressings to be adhesively attached to the skin with tape while still holding the dressing in position. This leads to quicker, less painful dressing changes, no leakage and more freedom to move.
Key Benefits
Key Benefits – The dressings and fastener
Indications
HidraWear is intended for wounds located in anatomical areas where adhesive dressings are challenging to apply or maintain, including:
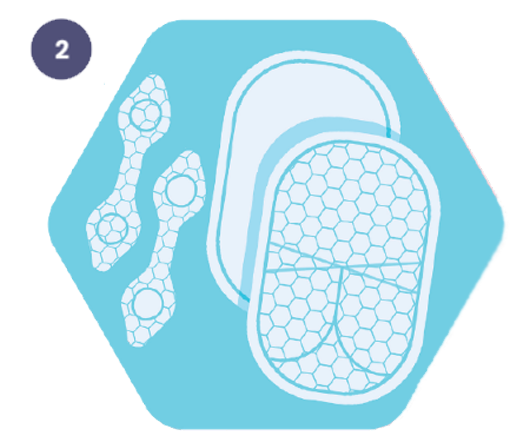
Dressings:
Contraindications & Precautions
Application / How to Use
1. Use the sizing guide to determine the appropriate base layer. If between sizes, choose the smaller for better support.
2. Put on the HidraWear base layer over the area of the wound.
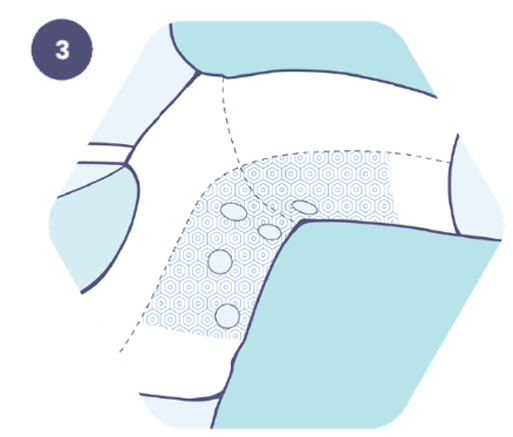
3. Insert the dressing through the functional window aligned with the wound site.
4. Place the dressing over the wound.
5. Secure the dressing using the SecureLock™ fastening tab that attaches to the dressing through the base layer.
6. To remove: detach the fastener, pull the base layer away from the skin, and slide out the dressing.
2. Put on the HidraWear base layer over the area of the wound.
3. Insert the dressing through the functional window aligned with the wound site.
4. Place the dressing over the wound.
5. Secure the dressing using the SecureLock™ fastening tab that attaches to the dressing through the base layer.
6. To remove: detach the fastener, pull the base layer away from the skin, and slide out the dressing.
Wear Time / Change Criteria
The dressing plus base layer system may remain in place until the primary dressing reaches its capacity, or signs of saturation, leakage, or wound healing changes warrant a change
Change earlier if the patient’s comfort, skin integrity, or wound exudate indicates replacement
Change earlier if the patient’s comfort, skin integrity, or wound exudate indicates replacement
Removal Instructions
1. Remove the fastening tab first
2. Gently peel the base layer away from the skin, supporting adjacent skin when necessary
3. Slide the dressing out through the window
4. Inspect peri-wound skin after removal
2. Gently peel the base layer away from the skin, supporting adjacent skin when necessary
3. Slide the dressing out through the window
4. Inspect peri-wound skin after removal
References
1. Moloney S., Fitzgerald D., Roshan D., Gethin G. (2022). Impact of hidradenitis suppurativa-specific wound dressing system on patient quality of life and dressing-related pain. Journal of Wound Care, 31(11), 898-906.
2. HidraWear. (2025). Official HidraMed Solutions website / product section.
3. HidraMed Solutions. (2021). HidraWear Prescriber’s Guide – UK.
2. HidraWear. (2025). Official HidraMed Solutions website / product section.
3. HidraMed Solutions. (2021). HidraWear Prescriber’s Guide – UK.
Ordering Details
HidraWear is available on prescription and can be dispensed by your local pharmacy. Alternatively, we have partnered with Bullen Healthcare, to provide a free home delivery service. For more information or to arrange an order call 0800 138 8171 or visit HidraWear – Bullen Healthcare