PolyMem® Non-Adhesive Dressings
A multifunctional polymeric membrane dressing without adhesive backing, combining cleansing, absorption, moisture balance, and antimicrobial protection (silver variant) for versatile use.
PolyMem® Non-Adhesive dressings integrate the core PolyMem® technology of cleansing, filling, and moisture management with the optional embedded silver, which releases ions in the presence of moisture to help reduce bioburden while minimising silver migration into the wound bed.¹acking
PolyMem® Non Adhesive Dressings
The optimal choice when cutting dressings to size and taping or tapeless is your preferred method of application. Use as a combined primary and secondary dressing or as a secondary dressing.
Product Benefits
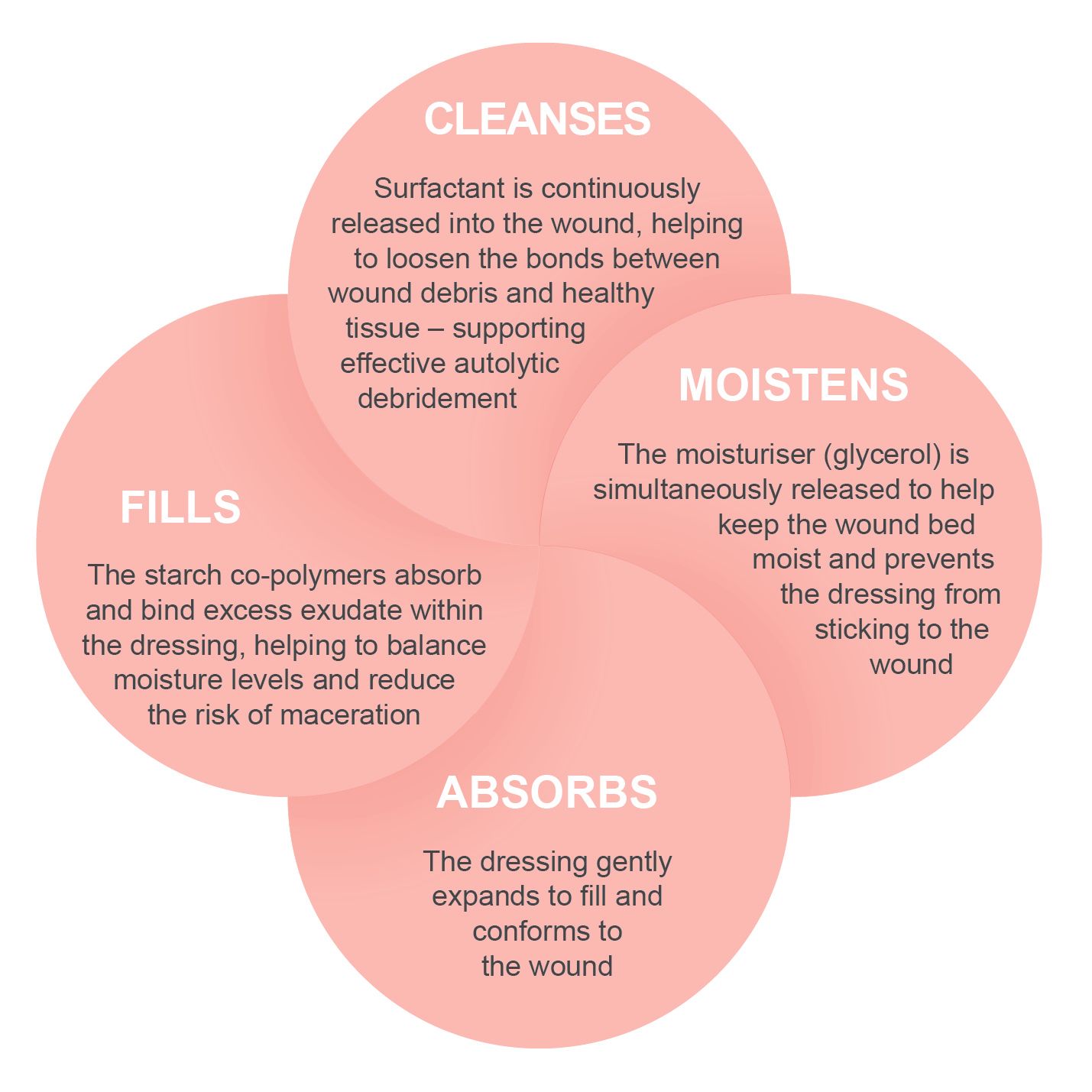
How the PolyMem formulation works:
All PolyMem® dressings effectively cleanse, fill, absorb and moisten wounds throughout the healing continuum.
They also:
References
Ferris Manufacturing Corporation. IFU-5930 PolyMem Pad or Roll Dressing (English).
2. PolyMem Non-Adhesive Dressings – Product Overview. WoundSource
2. PolyMem Non-Adhesive Dressings – Product Overview. WoundSource
Indications
When used under clinical supervision, PolyMem® Non-Adhesive dressings are suitable for a broad spectrum of wound types, particularly where infection risk or high bioburden is present. Examples include:
Contraindications & Precautions
Applying PolyMem® Non-Adhesive Dressings
1. Prepare the wound — cleanse and debride as per local protocol; ensure peri-wound skin is dry.
2. Select size — choose a dressing slightly larger than the wound to cover any inflamed area.
3. Apply — place the dressing directly over the wound bed, ensuring full contact.
4. Secure — as a non-adhesive format, use suitable fixation (e.g. tape, netting, gauze, bandage).
5. Outline — you may trace the wound margins on the dressing or over the cover dressing to help monitor healing.
6. Wear time & monitoring — may remain in place for up to 7 days, subject to exudate and clinical condition; change earlier if saturation or “strike-through” is visible.²
7. Removal — gently lift from one edge; if adhesion is encountered, moisten edges with sterile saline to ease removal.
Note: It is common to observe increased wound fluid in the initial days of application — this is part of the dressing’s moisture-attraction mechanism and indicates function.²
2. Select size — choose a dressing slightly larger than the wound to cover any inflamed area.
3. Apply — place the dressing directly over the wound bed, ensuring full contact.
4. Secure — as a non-adhesive format, use suitable fixation (e.g. tape, netting, gauze, bandage).
5. Outline — you may trace the wound margins on the dressing or over the cover dressing to help monitor healing.
6. Wear time & monitoring — may remain in place for up to 7 days, subject to exudate and clinical condition; change earlier if saturation or “strike-through” is visible.²
7. Removal — gently lift from one edge; if adhesion is encountered, moisten edges with sterile saline to ease removal.
Note: It is common to observe increased wound fluid in the initial days of application — this is part of the dressing’s moisture-attraction mechanism and indicates function.²
Ordering Details
PolyMem Non-Adhesive Dressings
The optimal choice when cutting dressings to size and taping or tapeless is your preferred method of application. Use as a combined primary and secondary dressing or as a secondary dressing.
The optimal choice when cutting dressings to size and taping or tapeless is your preferred method of application. Use as a combined primary and secondary dressing or as a secondary dressing.