PolyMem® WIC Cavity Fillers – Non-Adhesive
A Unique Multifunctional Polymeric Membrane Dressing, Designed To Facilitate Healing, Relieve Pain And Reduce Inflammation.
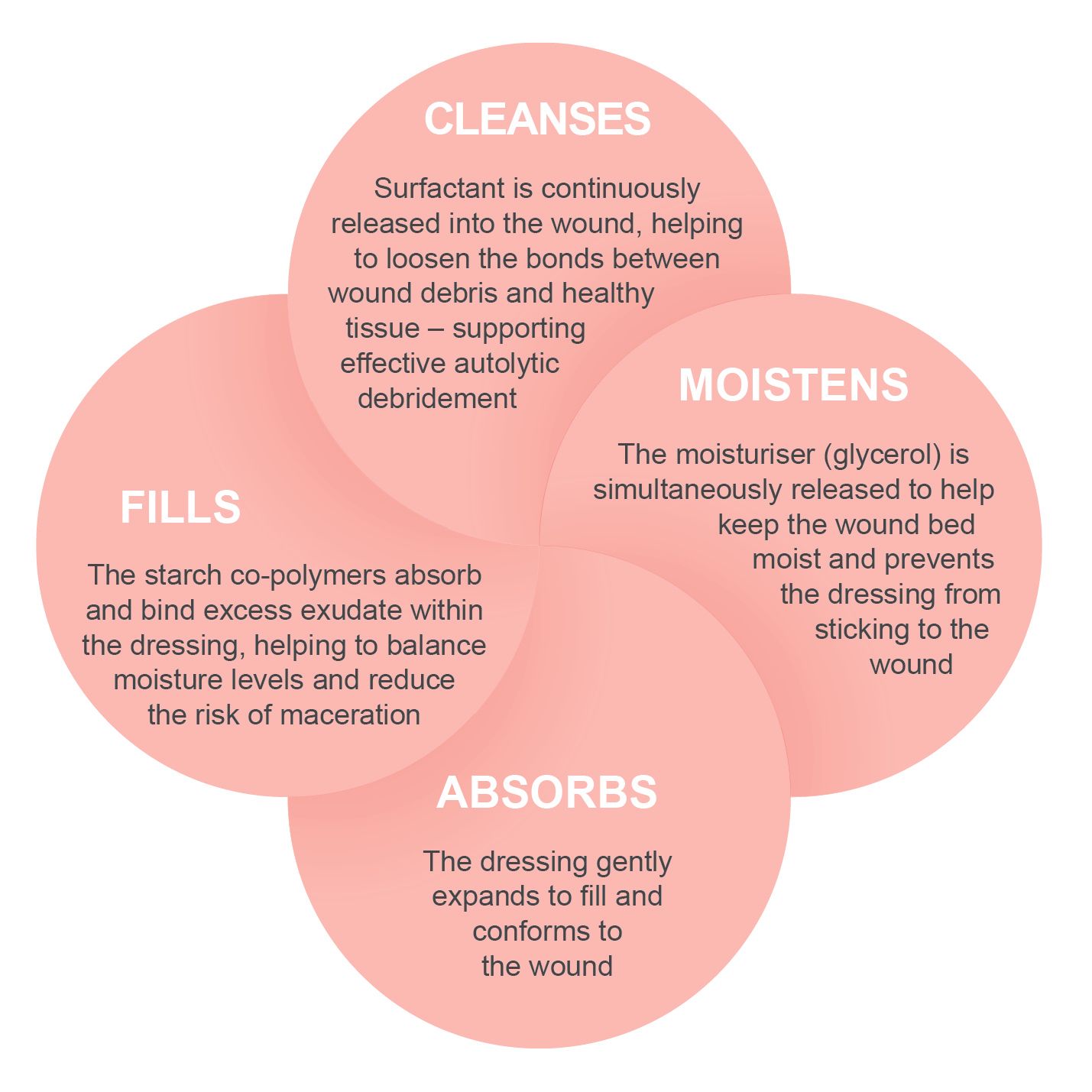
PolyMem® WIC Non-Adhesive Cavity Fillers are specifically developed to conform within wound cavities, tunnels, and undermined areas. They expand on contact with exudate, gently filling dead space while providing the proven multifunctional benefits of PolyMem® technology.
Product Benefits
The ONLY dressing of its kind…
When PolyMem® is applied to the wound, the dressing components work individually and synergistically to support wound healing and pain relief¹.
Note: An initial increase in wound exudate may be observed — this is a normal response and indicates the dressing is working.
References
1. Beitz A.J. et al. (2004). Polymeric membrane dressings with analgesic properties: clinical review.
2. Niezgoda J.A. et al. (2005). Pain reduction and wound healing with polymeric membrane dressings.
3. PolyMem® WIC Non-Adhesive Cavity Filler Instructions For Use, Ferris Mfg. Corp., 2025.
Indications
PolyMem WIC Non-Adhesive Cavity Fillers are intended for use by healthcare professionals in the management of cavity or tunnelling wounds with moderate to heavy exudate.
Typical indications include:
Contraindications & Precautions
Applying PolyMem® Wic Cavity Fillers
1. Prepare the wound — cleanse and debride as required; ensure peri-wound skin is dry.
2. Select size — choose a filler slightly smaller than the wound cavity; WIC expands by ~30 % when absorbing exudate.
3. Insert filler — place gently into the cavity or tunnel, ensuring contact with the wound bed but without tight packing.
4. For tunnelling wounds — use the rope (cord) variant, leaving one end visible for removal.
5. Cover with secondary dressing — apply an appropriate cover (e.g. PolyMem pad, foam, or film) to hold the filler in place.
6. Wear time — may remain in place up to 7 days depending on exudate and clinical condition. Change sooner if saturation occurs. 7. Removal — withdraw gently; if adherence is noted, moisten with sterile saline to ease removal.
NOTE: a dramatic increase in wound fluid may be observed during the first few days due to the fluid attraction. This is not uncommon and indicates that the dressing is working.
Ordering Details